Spinal Injury Project
Nerve Bridge Transplantation and Rehabilitation Human Clinical Trial
In partnership with

Expressions of Interest Now Open
Expressions of Interest are now open for Griffith University’s human clinical trial to test the olfactory cell transplantation therapy combined with long-term intensive rehabilitation for treating chronic traumatic spinal cord injury.
The Spinal Injury Project at Griffith University, in partnership with the Perry Cross Spinal Research Foundation, has recently started a world-first Nerve Bridge Transplantation and Rehabilitation Human Clinical Trial. The Spinal Injury Project is part of the Clem Jones Centre for Neurobiology and Stem Cell Research and is directed by Professor James St John.
The Spinal Injury Project has a team of internationally recognised researchers based in state-of-the-art laboratories at the Gold Coast and Nathan campuses of Griffith University. The project team is a leading group of research specialists including bioengineers, medical doctors, biological scientists and educators working together to develop this breakthrough treatment. The research was first initiated by the late Griffith University Professor Emeritus, and 2017 Australian of the Year, Alan Mackay-Sim almost 20 years ago. He was a pioneer in stem cell research and was successful in taking cells from the olfactory (nasal) system, transplanting them to the injury site and demonstrating that it was safe for use in humans.
Building on Professor Mackay-Sim’s incredible legacy, the ongoing research at Griffith University has made considerable improvements by developing a nerve bridge which improves how the cells are transplanted. The team has shown that the nerve bridges can repair spinal cord injury in preclinical work, and the clinical trial hopes to show that the nerve bridges can repair spinal cord injuries in humans.
The Spinal Injury Project is translating a comprehensive cell transplantation therapy with an exercise-based rehabilitation therapy to treat traumatic spinal cord injury. This world-first treatment for spinal cord injury uses specialised cells from within the nose. The cells are called olfactory ensheathing cells (OECs) and they can work in numerous different ways to stimulate repair of nerves. The olfactory cells are purified from a simple biopsy taken from within the nose, a cellular nerve bridge of OECs is manufactured for transplantation into the injured spinal cord, where the special regenerative capacity of transplanted OECs stimulates spinal cord regeneration.
The therapy has been developed over the last 10 years by a large team at the Clem Jones Centre for Neurobiology and Stem Cell Research, Griffith University in Queensland. The diverse team has over 40 translational biomedical researchers who come from 19 different countries. In addition to the lab members, the team also works with many external specialist researchers, clinicians and allied health professionals to drive the therapy to clinical trial.
The Foundation is proud to partner with Griffith University on this exciting endeavour with the support of our wonderful donors and supporters.
The Treatment
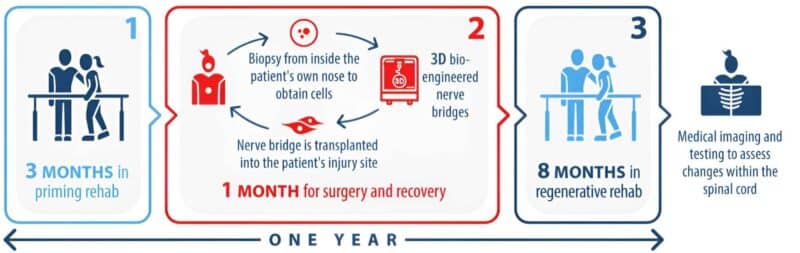
This ground-breaking, world-first treatment involves the transplantation of the patient’s own olfactory cells from the nose into the spinal cord. The cells are formulated into nerve bridges which are transplanted directly into the injury site. To help stimulate regeneration and to reinforce connections that are made, intensive rehabilitation takes place before and after the cell transplantation.
The human clinical trial, starting this year, aims to test the safety and efficacy of the nerve bridge transplantation and rehabilitation. The trial will have thirty participants, twenty who will undergo the nerve bridge transplantation and rehabilitation program (outlined below) and ten participants who do only the rehabilitation program.
The Cost
The Nerve Bridge Transplantation and Rehabilitation Human Clinical Trial is a truly inspirational endeavour, at a total cost of $15.3M. Through generous supporters like you, our funding partners, philanthropists, and our incredible fundraising community, the Foundation helped raise the initial $8.5M needed to commence the trial for 15 participants. This included a $2M contribution from Queensland Health and $1M from Nicola and Andrew Forrest.
Professor James St John and his team were also awarded a Medical Research Future Fund Stem Cell Therapies Grant of $6.8M for the clinical trial. With this funding the clinical trial was expanded to thirty participants.
Rehabilitation Trials >
To de-risk the trial, the Foundation with the support of our incredible donors, has already funded two rehabilitation clinical trials run by Griffith University and purchased an advanced LiveCyte microscope for the safety testing of the cells in preparation for the trial.

- The first rehabilitation trial involved people who had previous experience with rehabilitation programs but not at the intensity that is needed for the trial. The outcomes showed that the intensive long-term rehabilitation program is safe and that participants enjoyed the program and peer support. The Foundation fully funded this rehabilitation trial at a cost of $450,000 for five participants – read more here.
- The second rehabilitation trial showed that people with little experience with intensive rehabilitation were also able to safely complete the program and they too enjoyed the peer support and experience. Together these trials provided confidence that intensive long-term rehabilitation is suitable for people living with chronic spinal cord injury. The Foundation fully funded this second rehabilitation trial at a cost of $420,000 for five participants – read more here.
- Safety is a priority for the trial and for this reason the Foundation funded the LiveCyte microscope for the research team. This microscope uses live-cell imaging to track the fate of all cells that are being viewed. This allows the researchers to confirm that the cells are behaving in the appropriate way and that there are no undesirable cells within the population. This new technology provides an incredible advance for cell transplantation therapies and to have it as part of the trial is of immense importance for safety screening. The livecyte microscope was funded in full by the Foundation through the support of our donors at a cost of $400,000 – read more here.
Learn more on a virtual or in-person Tour >
We hold regular tours of our research lab on the Gold Coast, available to the public on a monthly basis. Learn more about attending an in-person tour or watch our most recent virtual tour via the button below!
Are you interested in participating in the upcoming clinical trial?
Expressions of Interest for the human clinical trial for olfactory nerve bridge cell transplantation combined with intensive exercise therapy are now open.
To learn more on the current criteria and register your interest to participate, please visit the Griffith University website.



